【Brief Paper Note】An electronic neuromorphic system for real-time detection of high frequency oscillations (HFO) in intracranial EEG
Paper Information
Title: An electronic neuromorphic system for real-time detection of high frequency oscillations (HFO) in intracranial EEG [1]
Authors: Mohammadali Sharifshazileh, Karla Burelo, Johannes Sarnthein, Giacomo Indiveri
Year: 2021
DOI: 10.1038/s41467-021-23342-2
Citation: Sharifshazileh, M., Burelo, K., Sarnthein, J.et al.An electronic neuromorphic system for real-time detection of high frequency oscillations (HFO) in intracranial EEG.Nat Commun12**, 3095 (2021). https://doi.org/10.1038/s41467-021-23342-2
Brief Introduction
High Frequency Oscillations (HFO) phenomenon in the context of EEG signal processing tasks refers to the observation of EEG at $80$-$500$ Hz from brain activity [1].HFO has shown a correlation with seizures in epileptic disorders, and has been utilized in several applications as a detection of epileptogenic zone as a kind of biomarkers.
In biological neural networks, the membrane potential of nerve cells changes over time and generates action potentials (spikes) under certain conditions. These spikes propagate through the synapse to the postsynaptic neuron. Artificial neural networks that simulate such behavior are also known as Spiking Neural Network (SNN).
Neuromorphic Computing simulates spiking neural networks. To accomplish these simulations more efficiently, SNNs are often deployed in Neuromorphic Hardware.
Based on the motivation to detect HFOs in real-time, and the realization that mixed-signal neuromorphic circuits offer the possibility to build compact and low-power neural network processing systems, this work designs and implements a neuromorphic system for Intracranial Electroencephalography (iEEG) for the HFO real-time detection task. The results demonstrate the feasibility of utilizing mixed-signal neuromorphic computing techniques to identify iEEG HFOs in real time.
Figure 1 demonstrates the overall image of automated HFO detection from iEEG using SNN.
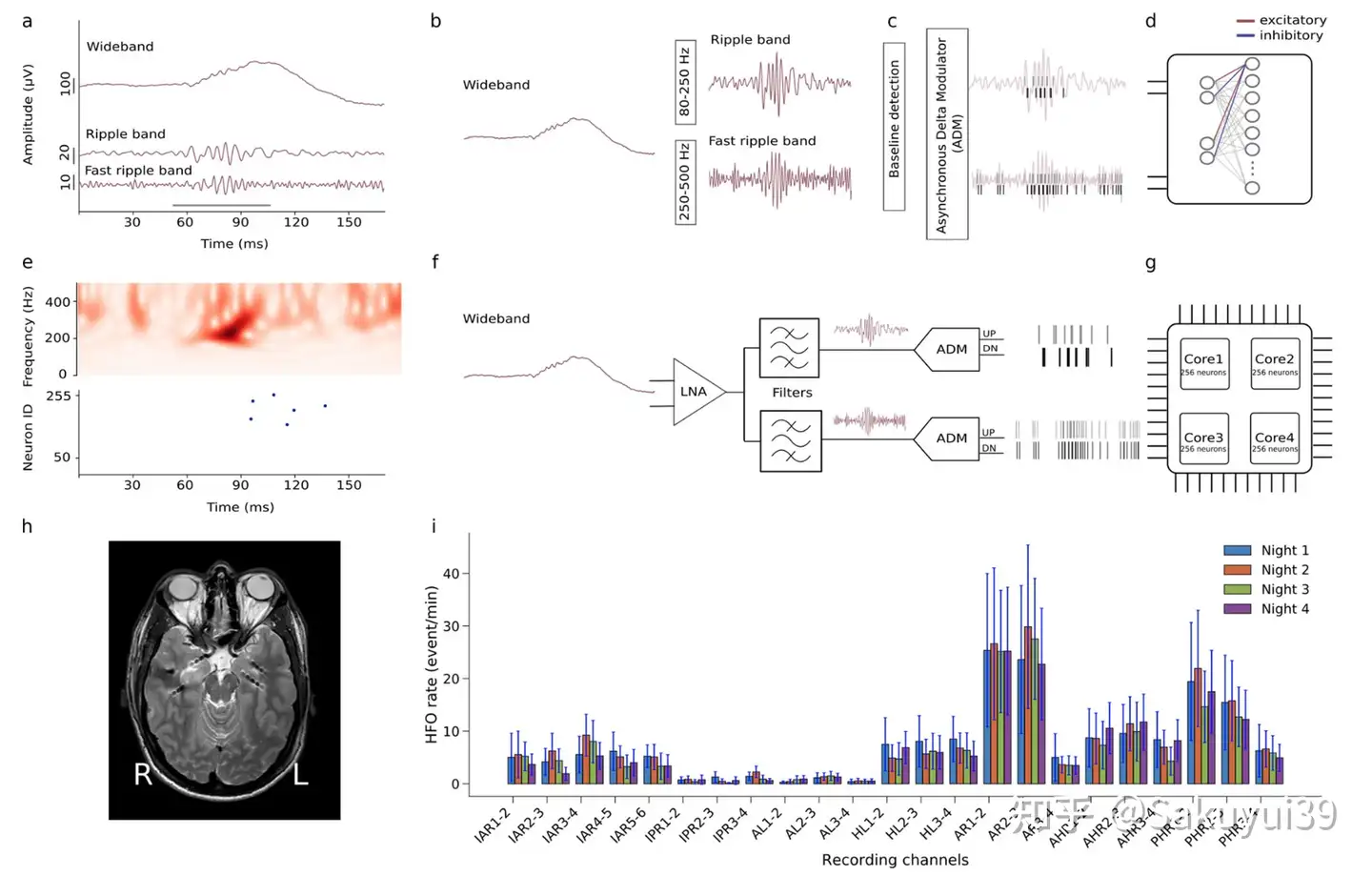
Fig. 1. Automated high-frequency oscillation detection using a bio-inspired spiking neural network (original Fig. 1)
The subplots of Fig. 1 read as follows:
(a)
Figure a shows pre-recorded iEEG signals in the wideband, ripple band ($80$-$250$ Hz) and fast ripple band ($250$-$500$ Hz). Cycles marked by gray bars represent clinically relevant HFOs.
(b-d)
Figures b-d show the software-simulated spiking neural network.
In the preprocessing stage, the wideband EEG signal is filtered in ripple bands and fast ripple bands.
The Baseline detection phase searches for the optimal threshold to apply to the asynchronous delta modulator (ADM).The ADM converts the signal into spikes.
The signal trajectory is encoded by UP spikes (gray bars) and DOWN spikes (black bars), which are then used as inputs to the SNN.
The SNN was implemented as a two-layer spiking network with dynamic synapses using Integrate and Fire neurons. Each neuron in the second layer receives four inputs: two columns of excitatory spikes from the UP channel and two columns of inhibitory spikes from the DOWN channel.
Network parameters were selected to demonstrate relevant temporal dynamics and neurons were tuned to produce output spikes that responded to input spike column patterns encoding clinically relevant HFOs.
(e)
e Top: Time-frequency spectrum of the iEEG fast ripple bands of Figure a.
e Bottom: SNN neuron issuance indicating the onset of HFO.
(f)
Figure f shows a block diagram of the input front end of the neuromorphic system.
The front-end consists of a Low Noise Amplifier (LNA), two configurable bandpass filters, and two ADM circuits.
(g)
The spikes generated by the ADM are sent to the multicore silicon neuron array configured to achieve the desired SNN.
(h)
MRI images of $7$ implanted depth electrodes, sampling medial temporal lobe structures in a patient with drug-resistant temporal lobe epilepsy (Patient 1).
(i)
HFO rates detected by the SNN Neuromorphic System for the four nocturnal recordings of Patient 1. Standard error bars show the variability of HFO rates over the nightly interval.
Recording channels AR$1$-$2$ and AR$2$-$3$ in the right amygdala showed the highest HFO rates and remained stable over multiple nights. Thus, the neuromorphic system predicted that therapeutic resection including the right amygdala would achieve seizure freedom. Indeed, resection including the right amygdala achieved seizure freedom for more than one year.
[Reflections]
This work demonstrates the possibility of applying neuromorphic computing algorithms as well as hardware for real-time detection of the properties of EEG signals. The low power overhead nature of neuromorphic computing hardware is advantageous for long-term real-time bio-signal monitoring and applications.
Deploying models for bio-signal parsing to neuromorphic hardware for real-time parsing maybe an interesting direction. For example, a traditional neural network model for bio-signal parsing could be converted to a SNN and then be deployed to customized hardware to simulate the SNN, or the SNN could be deployed on a more general neuromorphic hardware. It might also be interesting to exploit statistical properties of bio-signals with SNNs for efficient bio-signal analysis.
【Reference】
[1] Sharifshazileh, M., Burelo, K., Sarnthein, J.et al.An electronic neuromorphic system for real-time detection of high frequency oscillations (HFO) in intracranial EEG.Nat Commun12**, 3095 (2021). https://doi.org/10.1038/s41467-021-23342-2
[2] Park CJ, Hong SB. High Frequency Oscillations in Epilepsy: Detection Methods and Considerations in Clinical Application. J Epilepsy Res. 2019 Jun 30;9(1):1-13. doi: 10.14581/jer.19001. PMID: 31482052; PMCID: PMC6706641.